
Interesting links
Introduction
1. Selenoproteins
Selenoproteins are proteins that have a Selenocysteine in its sequence, a rare aminoacid that is a cysteine analog with selenium instead of sulfur and is coded by a UGA codon. Interestingly this codon, has second function, being a STOP codon in the standard genetic code. Therefore, specific machinery is needed for the recoding of the UGA codon to Selenocystein, in which SECIS (SElenoCysteine Insertion Sequence), a RNA secondary structure element present in the 3' UTR of eukaryotic selenoprotein gene transcripts, plays a key role. Due to this two roles of the UGA codon there are a lot of incorrectly annotated selenoprotein genes. Moreover, selenoproteins have a wide variety of functions, mostly catalyze redox reactions, but still unknown for many families.
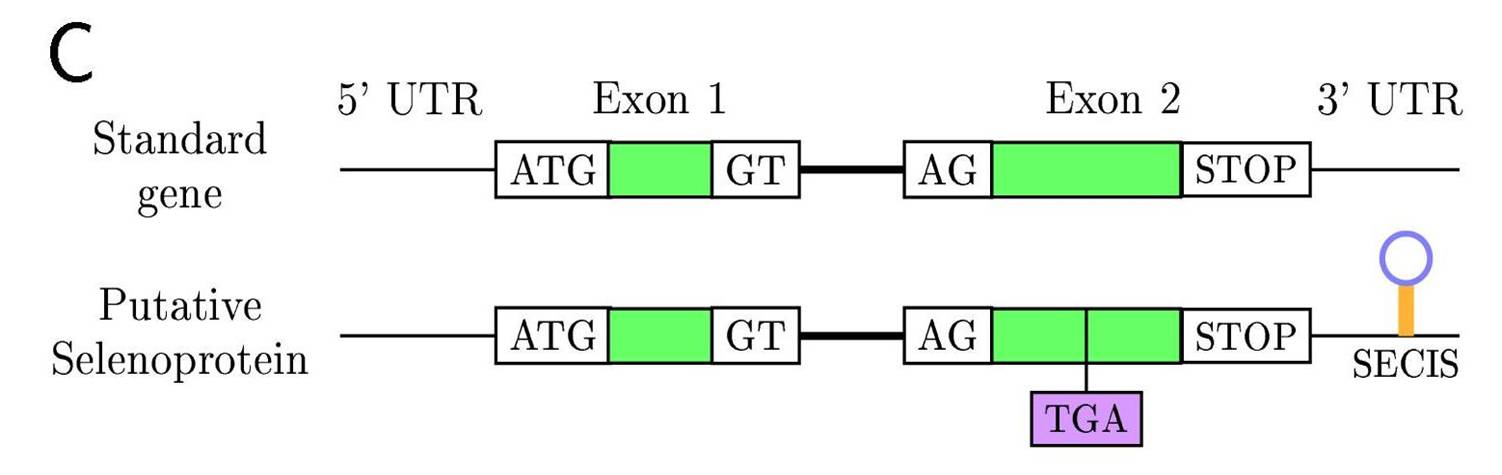
Standard gene and Selenoprotein comparision
1.1. Philogeny and Families
Selenoproteins are present in Eukaryota, Prokaryota and Archaea but not in every specie. As a general overview, 25 selenoproteins have been identified in Homo sapiens using Selenoprofiles, 3 in Drosophila melanogaster and none in Saccharamyces cerevisiae, demonstrating important differences between organisms.
There are currently 21 known families of selenoproteins in higher eukaryotes: Glutathione Peroxidases (GPx), Iodothyronine Deiodinase (DI), Selenoprotein 15 (Sel15 or 15kDa), Fish selenoprotein 15 (Fep15), SelM, SelH, SelI, SelJ, SelK, SelL, SelN, SelO, SelP, SelR, SelS, SelT, SelU, SelV, SelW, Thioredoxin Reductases (TR), SelenoPhosphate Synthetase (SPS). Some of these families may contain more than one member in a given genome and sometimes the orthologue of a selenoprotein has a cysteine instead of selenocysteine.
1.2. Biosynthesis
The before metioned SECIS element, follows an aminoacid consensus and has a variable secondary structure.
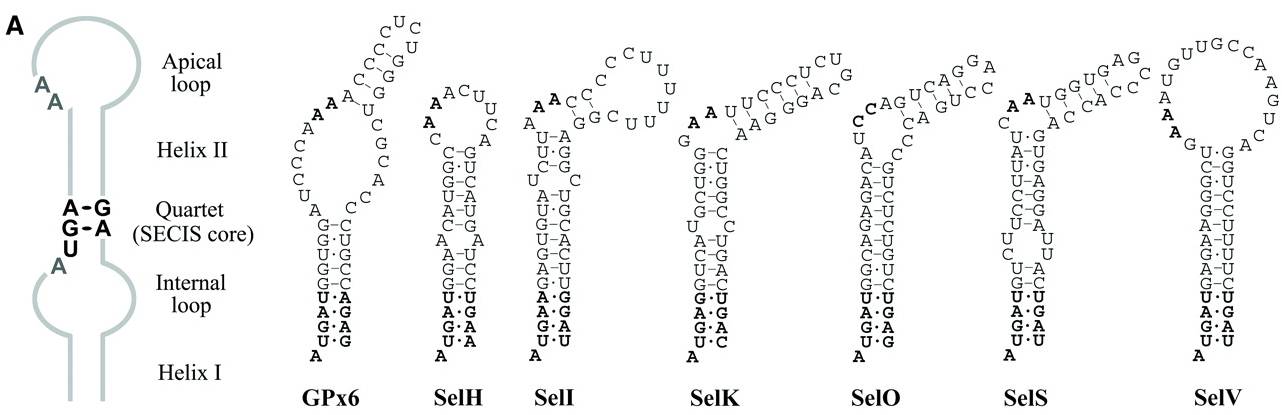
Mammalian SECIS element consensus and SECIS elements in selenoprotein genes.
In eukariota, SECIS recruits SECIS binding protein (SBP2) and it binds to the elongation factor of Sec (EFSec). EfSec approaches the selenocysteine-specific tRNA (tRNAsec) so the selenocysteine is added to the selenoprotein sequence. Translation goes on until a new STOP codon is found. Other proteins which participate specifically in this process are Selenophosphate Synthetase (SPS1 y SPS2), Selenocystein syntethase (SLA/LP), Selenocysteine associated protein (SECPP43) and Phosphoseryl tRNA Kinase (PSTK).
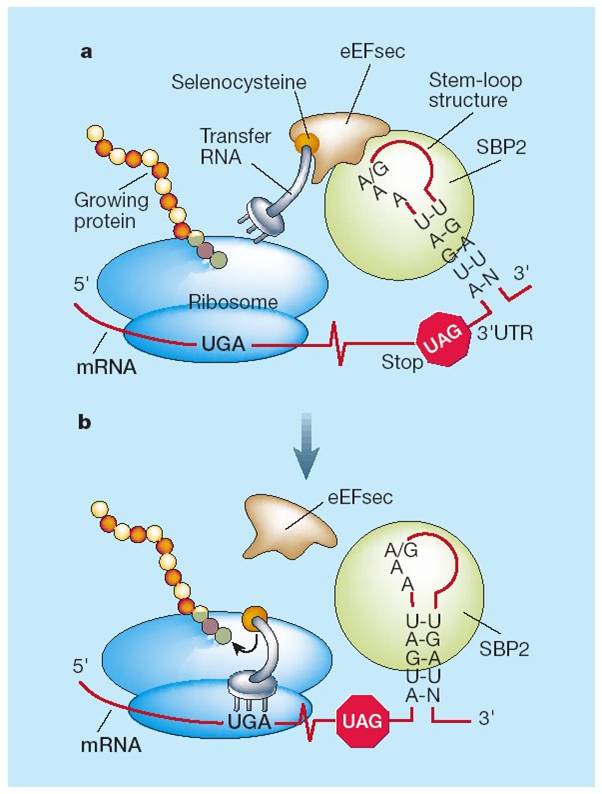
Selenoprotein machinery and biosynthesis
2. Latimeria chalumnae, a living fossil
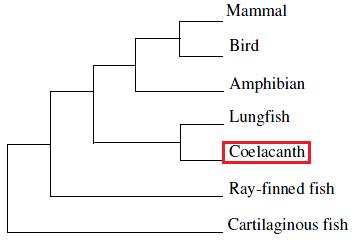
The genus Latimeria also known as coelacanth , encloses two different species L. chalumnae and L. meandoensis, which are lobe-finned fish related with lungfish and tetrapods.
It is considered a living fossil, it was thought to be extincted in the Late Cretaceous but was rediscovered in South Africa in 1938.
Latimeria chalumnae is piscivorous and nocturnal, its eyes have rods so they are acclimated to seeing in poor light. Coelacanth has 8 fins and can grow up to 1.8 meters. It has a unique locomotion, due to the high number of fins, it has high mobility and can orient its body in almost any direction. A remarkable feature of the coelacanth is its paired lobe fins that extend away from its body and move in an alternating pattern like legs.
3. Selenoproteins in L. chalumnae genome
L. chalumnae genome has not been annotated for SelenoProteins until now, therefore, the aim of this research study is to find all the selenoproteins present in the genome of this peculiar organism. Various philogenetic studies have demonstrated that L. chalumnae is the closest living relative of the land vertebrates, therefore is both related to ray-finned fishes (Actinopterygii) and to tetapods, such as amphibians. Therefore in this work, Danio rerio (Actinopterygii) and Xenopus leavis (Tetrapod) selenoproteins are used as querys in order to find selenoproteins in L. chalumnae genome.
Selenoproteins search was performed using different bioinformatic tools, such as tBLASTn, Exonerate, GeneWise, T_coffee and SECISearch. In addition, our analysis has been extended to include the search for the machinery involved in the synthesis of selenoproteins.
